Spotlights
Điều phối viên lâm sàng, Điều phối viên chương trình lâm sàng, Quản lý chương trình lâm sàng, Quản trị viên nghiên cứu lâm sàng, Điều phối viên nghiên cứu lâm sàng, Giám đốc nghiên cứu lâm sàng, Điều phối viên y tá nghiên cứu lâm sàng, Điều phối viên thử nghiệm lâm sàng, Quản lý thử nghiệm lâm sàng, Điều phối viên nghiên cứu
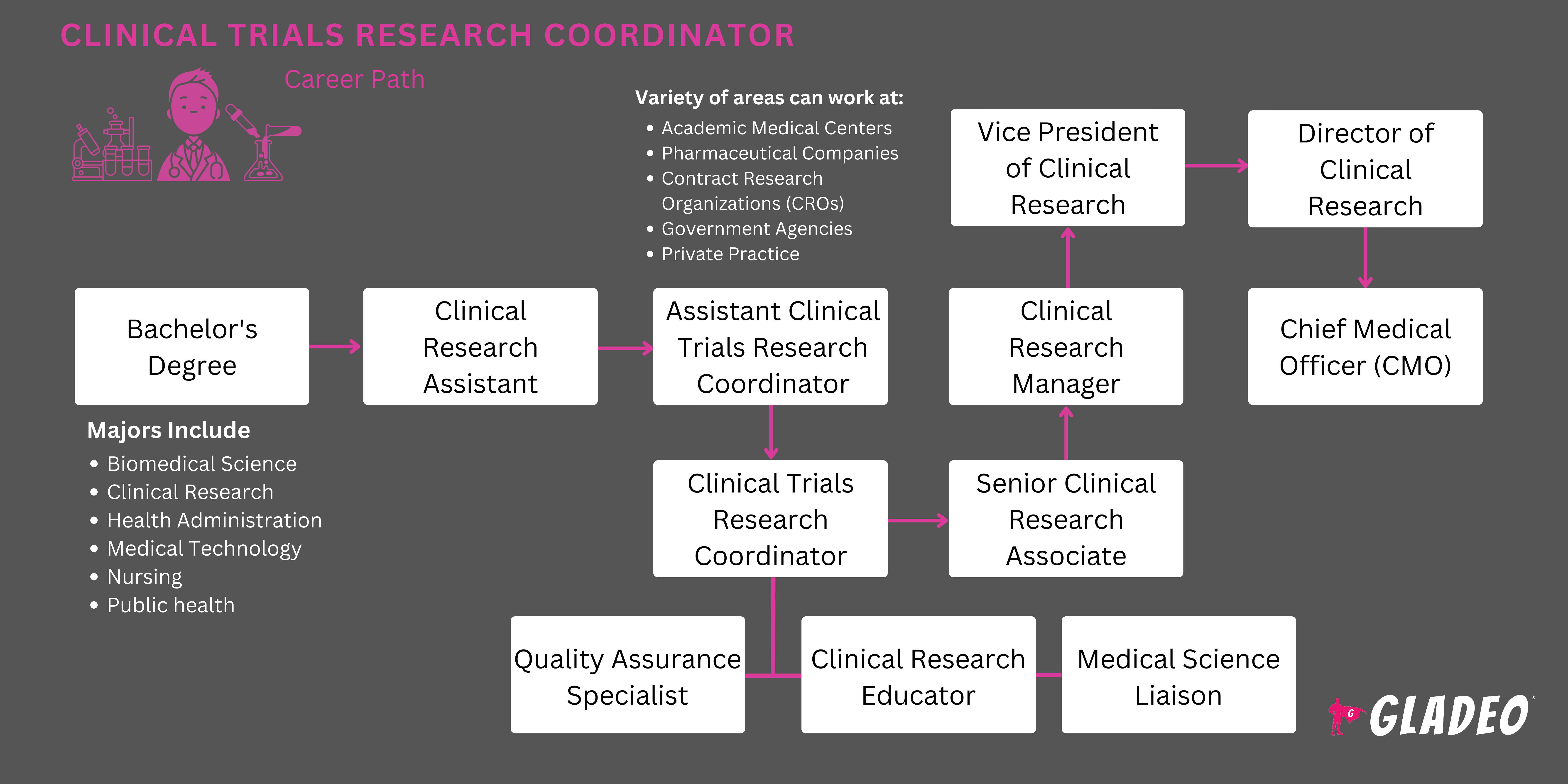
It takes several years and vast amounts of expensive research and development (R&D) to bring a new medical drug to market. This R&D is done at research sites where teams of experts are assigned to conduct trials and collect data to determine if a drug is effective and safe. At the heart of such operations are a principal investigator and a Clinical Research Coordinator (aka CRC).
CRCs manage several key aspects of the clinical trials process. Collaborating with other researchers and healthcare professionals, they establish trial protocols, recruit volunteers to participate, collect and manage data, prepare detailed documentation, and maintain compliance with regulatory standards.
Through meticulous planning, coordination, and execution of clinical trials, Clinical Research Coordinators help innovative medical solutions receive approval. Thus, their work directly contributes to the development of new treatments and therapies, making a significant impact on the healthcare industry and patient outcomes!
- Playing a pivotal role in the development of new therapies and treatments
- Advancing medical research to improve patient care and outcomes
- Contributing to the body of scientific knowledge in healthcare
Lịch làm việc
- Clinical Research Coordinators typically work full-time. Schedules may vary, with night or weekend work possible.
Nhiệm vụ tiêu biểu
- Oversee clinical trial operations from start to finish
- Collaborate with healthcare professionals and researchers to establish protocols, including administration and data collection guidelines
- Establish realistic goals for each trial phase
- Recruit and screen trial volunteers via interviews and medical record reviews
- Organize suitable study spaces, equipment, and supplies
- Schedule activities that volunteers need to complete
- Order, label, store, and ship collection samples
- Take volunteer vital signs and perform other procedures, as applicable
- Advise on dosage modifications based on unique patient attributes
- Collect, code, and analyze study trial data
- Manage documentation such as progress notes, side effect or adverse event reports, compliance forms, and other records
- Record and report adverse events to oversight agencies
- Ensure compliance with established protocols and regulatory guidelines
- Coordinate funding requests and manage budgets
- Provide support and updates to trial participants
- Support quality assurance evaluations. Address any trial-related problems
- Keep stakeholders updated on the progress
- Work with investigators to consolidate study findings for external presentation
Trách nhiệm bổ sung
- Review research journals and take professional development courses to keep up with advancements
- Train staff on policies and protocols
- Coordinate site visits and manage incoming questions
- Liaise with laboratories and investigators, as needed
- Create and distribute materials to solicit volunteers
- Administer trial medications or issue trial medical devices for participant use
- Share participant figures with statistical centers
Kỹ năng mềm
- Khả năng thích ứng
- Phân tích
- Chú ý đến chi tiết
- Hợp tác
- Tư duy phản biện
- Sự tò mò
- Suy luận suy diễn
- Sự siêng năng
- Ethical judgment
- Độc lập
- Kỹ năng giao tiếp và ứng xử giữa các cá nhân
- Khả năng giải quyết vấn đề
- Lập kế hoạch chiến lược
- Làm việc theo nhóm
- Quản lý thời gian
Kỹ năng kỹ thuật
Clinical Research Coordinators need technical skills related to the below:
- Clinical data management systems and software (e.g., Oracle Clinical, Medidata Rave, and SAS)
- Clinical trial management software (e.g., Oracle Clinical, Medidata Rave)
- Data interpretation
- Data privacy and security practices (such as encryption, access controls, and audit trails)
- Electronic health records
- Healthcare regulations and compliance standards
- Institutional Review Board submissions
- Knowledge of Good Clinical Practice guidelines
- Medical terminology and clinical procedures understanding
Project management - Regulatory compliance and documentation
- Reporting tools (like SPSS and R)
- Phân tích thống kê
- Working with regulatory agencies
- Academic research institutions
- Công ty công nghệ sinh học
- Contract research organizations
- Government health agencies
- Công ty dược phẩm
Clinical Research Coordinators should love research and be committed to developing more effective interventions such as pharmaceuticals and medical technologies.
Their work demands vigilance, precision, and the ability to manage complex logistics. But these professionals don’t just deal with equipment and data. They’re dealing with real patients who volunteered to be part of an experimental testing process! Oversights or errors could put volunteers at risk, so clinical trials must be conducted efficiently, ethically, and with special attention to participant safety.
The world of clinical research is embracing technological advancements like electronic data capture systems to streamline data collection and management, reducing errors and speeding up analysis times.
The use of real-world data and real-world evidence is revolutionizing study designs and providing deeper insights into treatment effectiveness and safety in real-world settings.
The trend of personalized medicine is also impacting clinical research due to its focus on targeted therapies based on individual genetic profiles. This requires more intricate study designs and specialized coordination but can lead to better patient outcomes and care paradigms.
Individuals attracted to this career likely had a keen interest in science and health from an early age. They may have enjoyed science fairs, running STEM-related experiments at home, or volunteering in healthcare settings.
- Clinical Research Coordinators usually need a bachelor’s degree in biology, nursing, public health, health sciences, microbiology, or a related area
○ In addition, relevant work experience in clinical research or healthcare is required
○ Many positions require a thorough background check that may include criminal history, employment history, drug screening, and a credit check
- Common college courses include:
○ Biomedical Ethics
○ Biostatistics
○ Clinical Data Management
○ Clinical Research Methods
○ Clinical Trial Design and Implementation
○ Epidemiology
○ Ethics in Clinical Research
○ Good Clinical Practice Guidelines
○ Health Economics and Outcomes Research
○ Patient Recruitment and Retention Strategies
○ Pharmacology
○ Quality Assurance in Clinical Research
○ Regulatory Affairs
○ Risk Management in Clinical Trials
- A popular, optional certification is the Association of Clinical Research Professionals’ Certified Clinical Research Coordinator (CCRC)
○ To sit for the CCRC exam, applicants need either:
◘ 3,000 hours of verifiable work experience related to human subject research, or;
◘ 1,500 hours of verifiable work experience and a clinical research degree from a CHEA-accredited institution
○ Other Association of Clinical Research Professionals credentials include:
◘ Certified Clinical Research Associate
◘ Certified Principal Investigator
- Optional certifications from other organizations include:
○ Society of Clinical Research Associates - Certified Clinical Research Professional
○ Public Responsibility in Medicine and Research - Certified IRB Professional
○ Research Administrators Certification Council - Certified Pre-Award Research Administrator
○ Research Administrators Certification Council - Certified Research Administrator
- Not all Clinical Research Coordinators hold a bachelor’s degree, but at least 60% do.
- Look for programs featuring:
○ Curricula that offer comprehensive coverage of clinical research principles;
○ Internship opportunities with hospitals, research institutions, or pharmaceutical companies;
○ Faculty with real-world experience in clinical trials and research;
○ High-quality facilities with the latest research tools and technologies;
○ Connections with the clinical research industry for potential employment opportunities!
- Consider the program's cost and the availability of financial aid or scholarships.
- Focus on STEM courses, especially biology, human anatomy and physiology, microbiology, genetics, chemistry, organic chemistry, biochemistry, algebra, geometry, pre-calculus or calculus, statistics, physics, health sciences, biotechnology, computer science, data science, and psychology
- Knock out Advanced Placement courses to prepare for college
- Participate in science clubs, fairs, and research projects, particularly ones that may involve statistical software and data analysis
- Get a feel for the subject matter through short introductory courses via Coursera, Udemy, edX, and other sites
- Volunteer or seek part-time work in healthcare settings to understand patient care
- Engage in internships or job-shadowing opportunities in clinical research settings
- Follow clinical research news, blogs, and journals to stay informed on industry trends
- Network with professionals in the field for guidance and mentorship
- Keep a record of your achievements, projects, and skills for your resume
- Decide who you want to serve as your references. Ask for their permission to give out their contact information
- Hãy đến trung tâm hướng nghiệp của trường để được trợ giúp chuẩn bị sơ yếu lý lịch và thực hiện phỏng vấn thử
- Apply for entry-level positions, volunteer roles, internships, work placements, or clinical research apprenticeships to gain real-world experience
- Review job postings on Indeed, Glassdoor, and other portals
- Note the keywords in job ads. Try to incorporate them into your resume where possible
- Common keywords might include:
○ Adverse Event Reporting
○ Budget Management
○ Clinical Protocols
○ Clinical Research
○ Clinical Trial Management Systems
○ Data Collection Methods
○ Electronic Data Capture Systems
○ Ethical Standards
○ Informed Consent Process
○ IRB Submissions
○ Patient Screening
○ Pharmacovigilance (i.e., drug safety)
○ Protocol Development
○ Quality Assurance
○ Regulatory Compliance
○ Study Design and Implementation
- Take a look at the Clinical Research Coordinator resume templates for ideas
- Review Clinical Research Coordinator interview questions such as “Have you ever made a mistake with entering data into a database? How did you realize it, and what did you do to correct it?”
- Practice your replies in mock interviews with friends
- Be familiar with the clinical research technologies, terminology, and trends
- Dress appropriately for interviews!
- Seek advice from mentors and supervisors about career progression
- Continue learning through professional development courses or by earning certifications like the Association of Clinical Research Professionals’ Certified Principal Investigator
- Consider knocking out a graduate degree
- Volunteer to take on more complex simulation projects and leadership roles
- Build relationships with fellow researchers and healthcare professionals
- Specialize in an emerging area of clinical research like gene therapy
- Stay active in professional organizations such as the Association of Clinical Research Professionals
- Contribute to forums, blogs, and journals. Present at seminars and conferences
- If needed to advance, consider relocating to look for jobs at larger organizations
Các trang web
- Hiệp hội Quản lý Thông tin Y tế Hoa Kỳ
- American Medical Informatics Association
- American Statistical Association - Biopharmaceutical Section
- Association of Clinical Research Professionals
- Association of Professional Healthcare Analysts
- Biocomplexity Institute of Virginia Tech
- Tin sinh học
- BioInformatics Inc.
- Clinical Data Interchange Standards Consortium
- Clinical Research: Open Access
- Clinical Research Society
- Clinical Trials
- ClinicalTrials.gov
- Commission on Accreditation for Health Informatics and Information Management Education
- Genedata
- Healthcare & Data Analytics Association
- Hiệp hội hệ thống quản lý và thông tin chăm sóc sức khỏe
- Health Data Science
- HLA Informatics Group
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
- International Society for Computational Biology
- International Society for Pharmacoeconomics and Outcomes Research
- International Society of Clinical Biostatistics
- Journal of Pre-Clinical and Clinical Research
- Journal of the Society for Clinical Data Management
- Mathematical Biosciences Institute
- National Association of Health Data Organizations
- National Center for Biotechnology Information
- National Center for Genome Resources
- Hiệp hội chăm sóc sức khỏe quốc gia
- Viện Khoa học Y khoa Tổng quát Quốc gia
- Viện Y tế Quốc gia
- NIH Intramural Research Program
- Open Bioinformatics Foundation
- Perspectives in Clinical Research
- Viện quản lý dự án
- Qlucore
- Seqera Labs
- Hiệp hội quản lý dữ liệu lâm sàng
- Society of Clinical Research Associates
- Society of Clinical Trials
- Strand Life Sciences
- Translational Genomics Research Institute
- Trials
Sách vở
- Essential Concepts in Clinical Research: Randomised Controlled Trials and Observational Epidemiology, by Kenneth Schulz PhD and David Grimes MD
- Foundations of Clinical Research: Applications to Evidence-Based Practice, by Leslie Portney DPT PhD
- Principles and Practice of Clinical Research, by John Gallin MD (Editor), et. al.
Clinical Research Coordinators are essential to the process of conducting medical trials, but there is a limited number of positions available at any given time. If you’re interested in exploring some alternative career paths, consider the following options:
- Nhà thống kê sinh học
- Clinical Data Manager
- Clinical Nurse Specialist
- Data Manager for Clinical Research
- Health Informatics Specialist
- Health Specialties Teacher
- Medical and Clinical Laboratory Technologists
- Medical and Health Services Manager
- Nhà khoa học y tế
- Medical Writer
- Natural Sciences Manager
- Pharmaceutical Sales Representative
- Public Health Analyst
- Quality Assurance Analyst
- Regulatory Affairs Specialist
- Nhà khoa học nghiên cứu
- Social Science Research Assistant
- Chuyên gia tài nguyên nước
Nguồn cấp tin tức
Công việc nổi bật

Các khóa học và công cụ trực tuyến

Kỳ vọng về mức lương hàng năm
New workers start around $65K. Median pay is $79K per year. Highly experienced workers can earn around $100K.
Kỳ vọng về mức lương hàng năm
New workers start around $43K. Median pay is $55K per year. Highly experienced workers can earn around $71K.
Kỳ vọng về mức lương hàng năm
New workers start around $57K. Median pay is $80K per year. Highly experienced workers can earn around $85K.
Kỳ vọng về mức lương hàng năm
New workers start around $51K. Median pay is $65K per year. Highly experienced workers can earn around $83K.







